
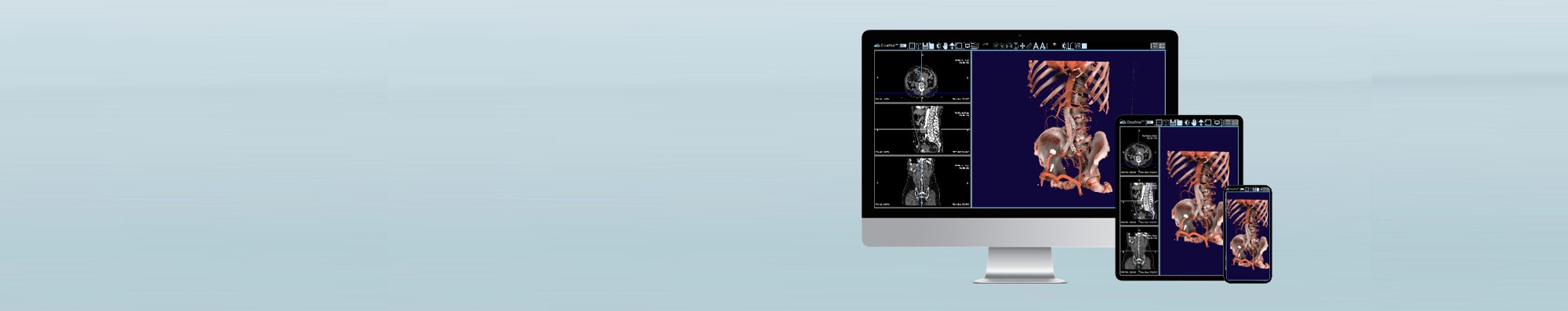
IMS CloudVue is one of the first cloud-native technologies to enable the remote, seamless, and instant view
of linked medical images from DICOM using the Medical Imaging Server for DICOM. CloudVue provides
healthcare clinicians and researchers the ability to view and share medical imaging data in a highly secure
cloud environment – anywhere they can get access to an internet connection.
Steven Borg
Director, Microsoft, Health Cloud and Data
IMS CloudVue
Finally, a native cloud-based viewing platform right at your fingertips.
• Security of a cloud storage solution.
• Performance of a workstation.
• Flexibility of a mobile app.
No installation. No setup. No registration necessary.
CHECK OUT OUR GALLERYWant a quick demo?TRY OUT OUR FREE DEMO

REAL-TIME VIEWING
ANYTIME. ANYWHERE. ANY DEVICE.






